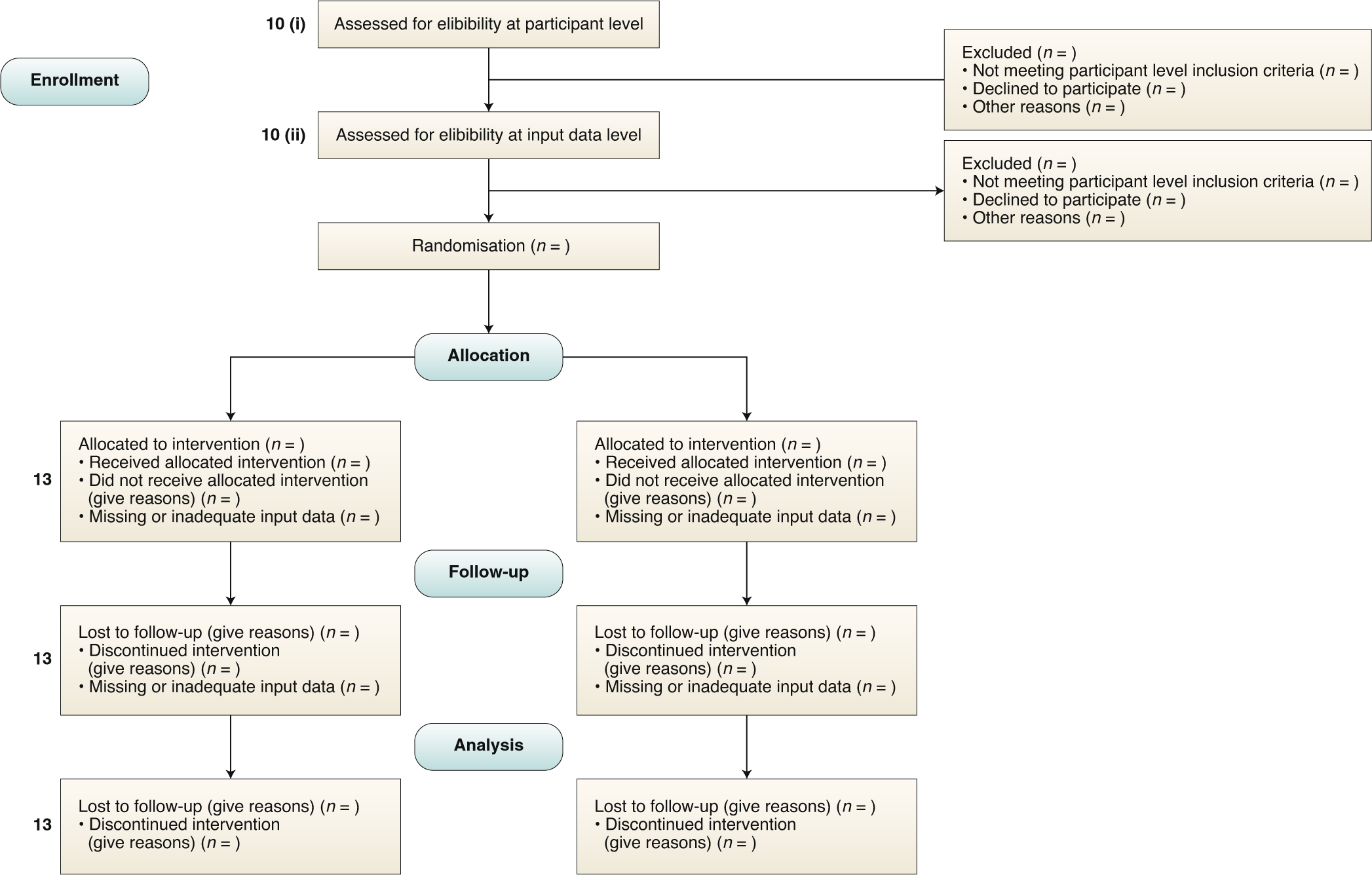
Early phase oncology clinical trials in Malaysia: current status and future perspectives - Voon - 2023 - Asia-Pacific Journal of Clinical Oncology - Wiley Online Library
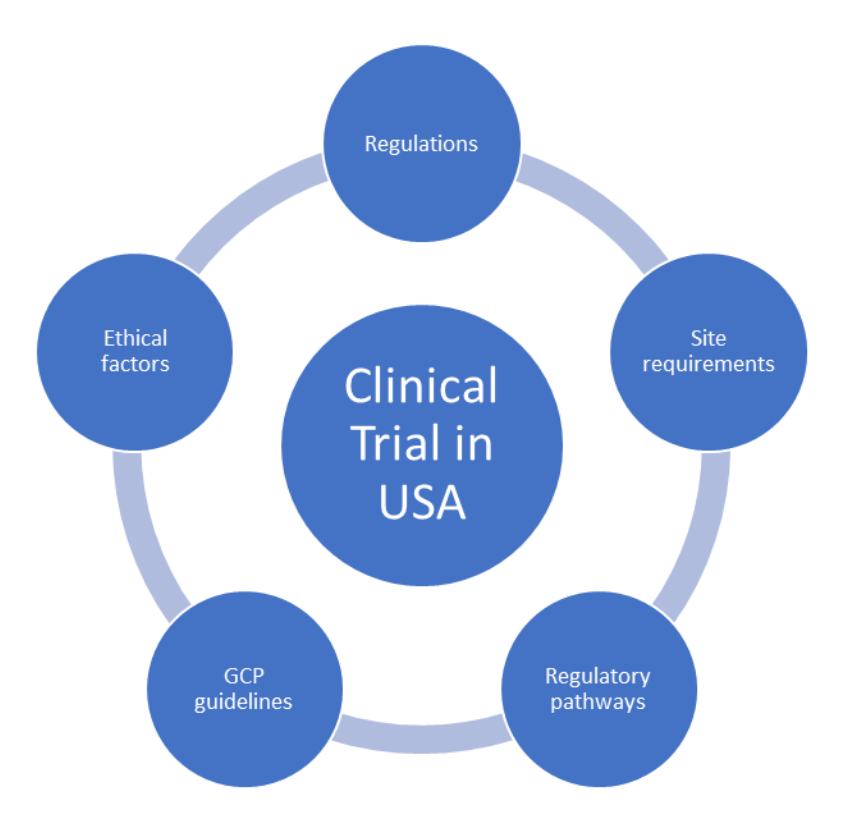
![PDF] RECENT ADVANCEMENT IN REGULATORY GUIDELINES FOR CLINICAL TRIALS IN USA AND INDIA | Semantic Scholar PDF] RECENT ADVANCEMENT IN REGULATORY GUIDELINES FOR CLINICAL TRIALS IN USA AND INDIA | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/52893bcfc57fecda0c60e34dd1fe46414c867575/9-Figure4-1.png)
PDF] RECENT ADVANCEMENT IN REGULATORY GUIDELINES FOR CLINICAL TRIALS IN USA AND INDIA | Semantic Scholar

When innovation outpaces regulations: The legal challenges for direct‐to‐patient supply of investigational medicinal products - Malone - 2022 - British Journal of Clinical Pharmacology - Wiley Online Library

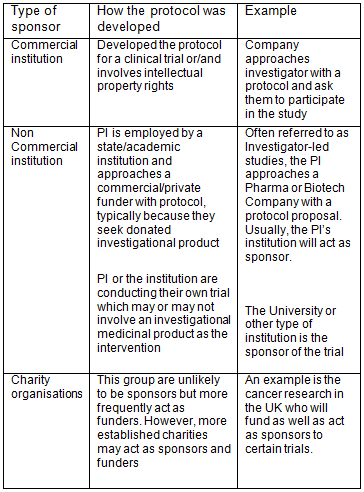
Comparison of clinical trial guidelines in USA, EU and India, Singapore. | Download Scientific Diagram
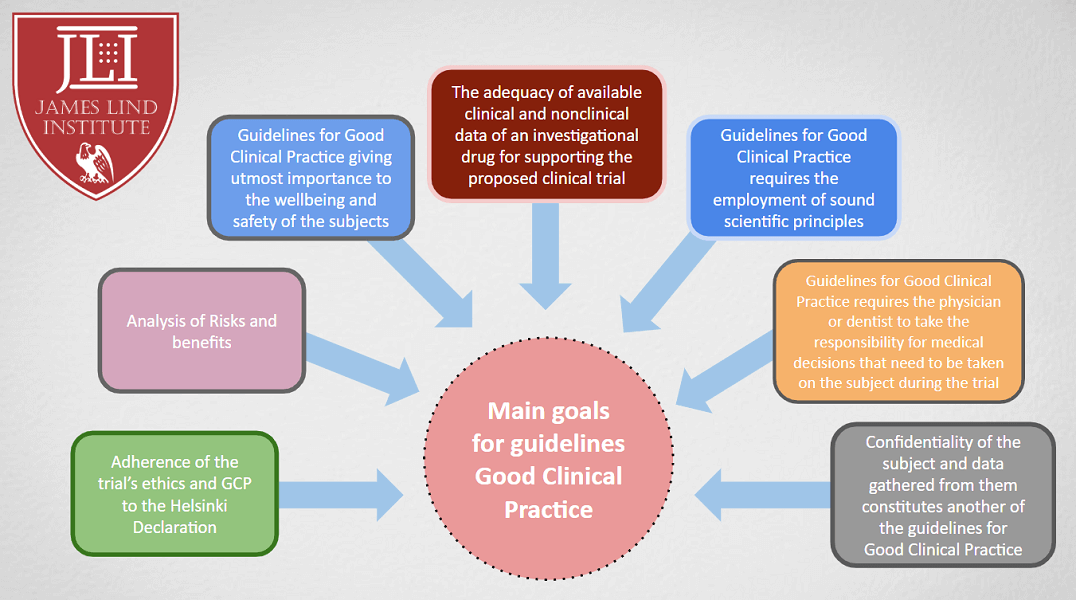
![PDF] RECENT ADVANCEMENT IN REGULATORY GUIDELINES FOR CLINICAL TRIALS IN USA AND INDIA | Semantic Scholar PDF] RECENT ADVANCEMENT IN REGULATORY GUIDELINES FOR CLINICAL TRIALS IN USA AND INDIA | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/52893bcfc57fecda0c60e34dd1fe46414c867575/2-Figure1-1.png)
PDF] RECENT ADVANCEMENT IN REGULATORY GUIDELINES FOR CLINICAL TRIALS IN USA AND INDIA | Semantic Scholar

Guidelines for cellular and molecular pathology content in clinical trial protocols: the SPIRIT-Path extension - The Lancet Oncology