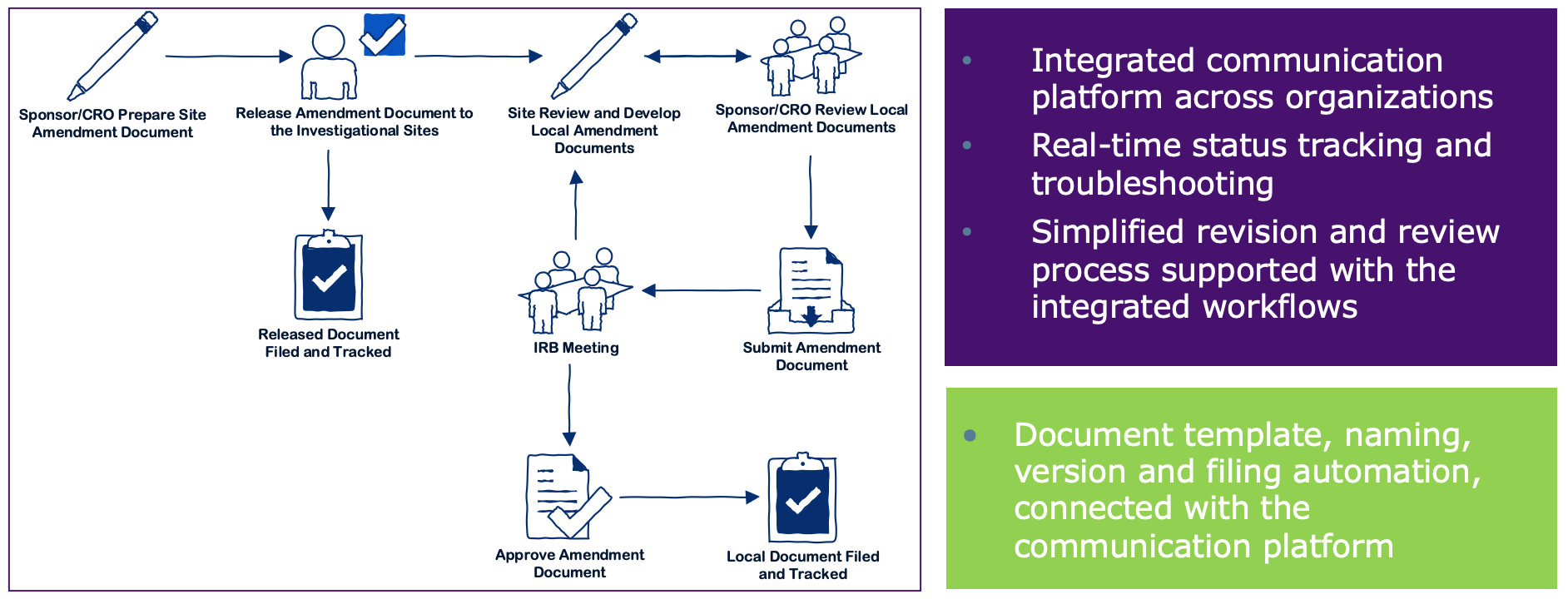
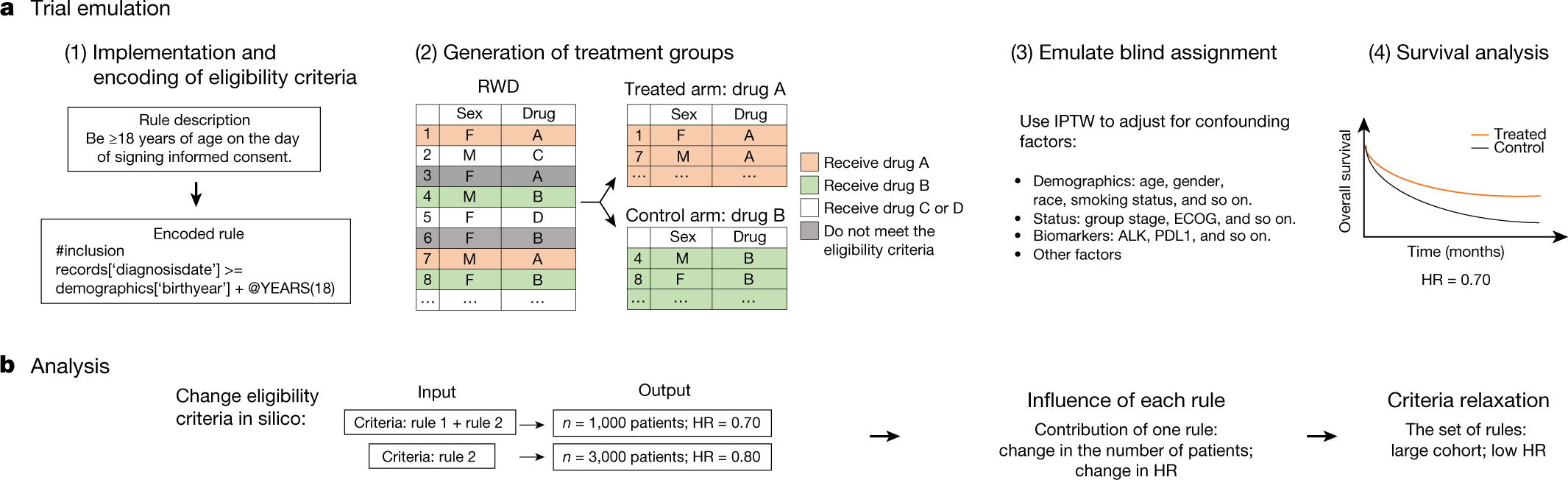
Recruitment of Black Adults into Cardiovascular Disease Trials | Journal of the American Heart Association

Reporting guidelines for clinical trial reports for interventions involving artificial intelligence: the CONSORT-AI extension - The Lancet Digital Health
FDA Guidance on Conduct of Clinical Trials of Medical Products during COVID-19 Pandemic: Guidance for Industry, Investigators, a

Early phase clinical trials extension to guidelines for the content of statistical analysis plans | The BMJ

Assessing the detection, reporting and investigation of adverse events in clinical trial protocols implemented in Cameroon: a documentary review of clinical trial protocols – topic of research paper in Clinical medicine. Download

Guidelines for clinical trial protocols for interventions involving artificial intelligence: the SPIRIT-AI extension - The Lancet Digital Health
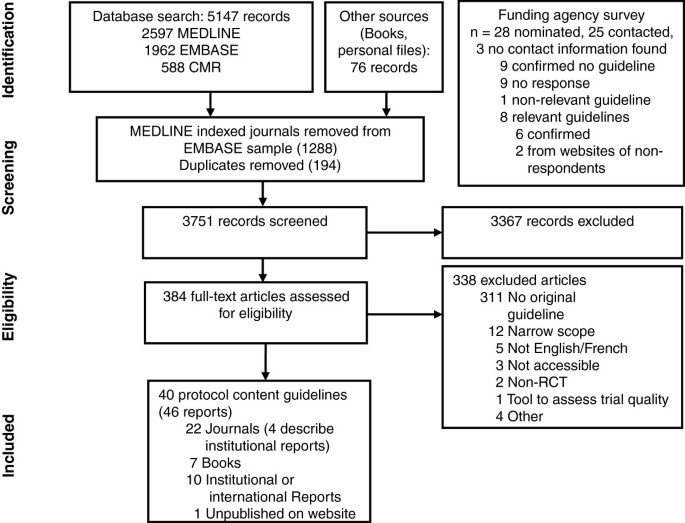
Guidelines for randomized clinical trial protocol content: a systematic review | Systematic Reviews | Full Text

Guidelines for clinical trial protocols for interventions involving artificial intelligence: the SPIRIT-AI extension - The Lancet Digital Health

Guidelines for clinical trial protocols for interventions involving artificial intelligence: the SPIRIT-AI extension - The Lancet Digital Health
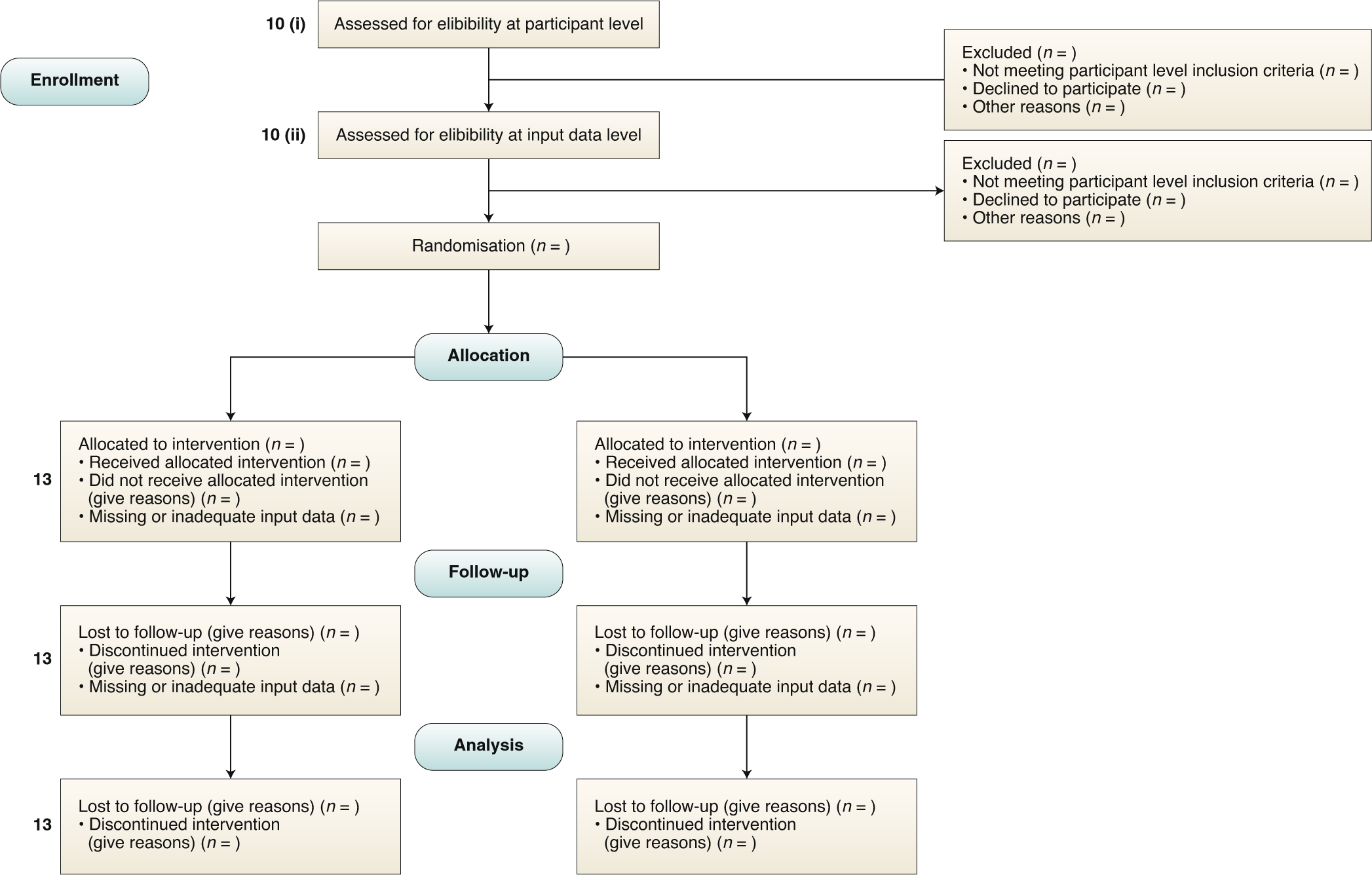
Guidelines for clinical trial protocols for interventions involving artificial intelligence: the SPIRIT-AI extension | Nature Medicine