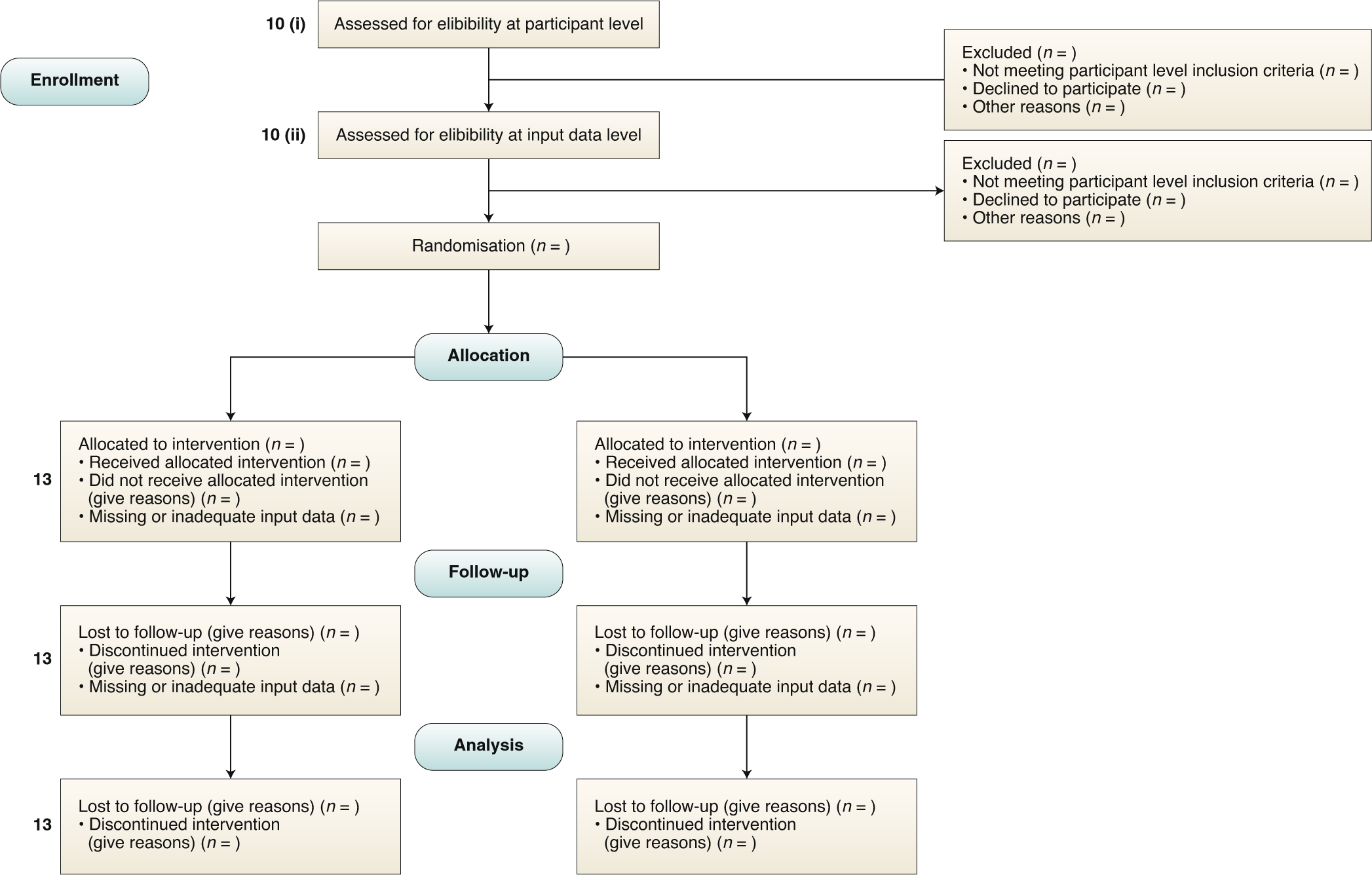
Guidelines for clinical trial protocols for interventions involving artificial intelligence: the SPIRIT-AI extension | Nature Medicine

PDF) Development and Implementation of Clinical Trial Protocol Templates at the National Institute of Allergy and Infectious Diseases
Research Study Protocol Template Instructions Study Protocol Title: Table of Contents: List of Abbreviations: Principal Investig













