Clinical trial protocol components onboarding mobile app page screen with concepts 2283568 Vector Art at Vecteezy
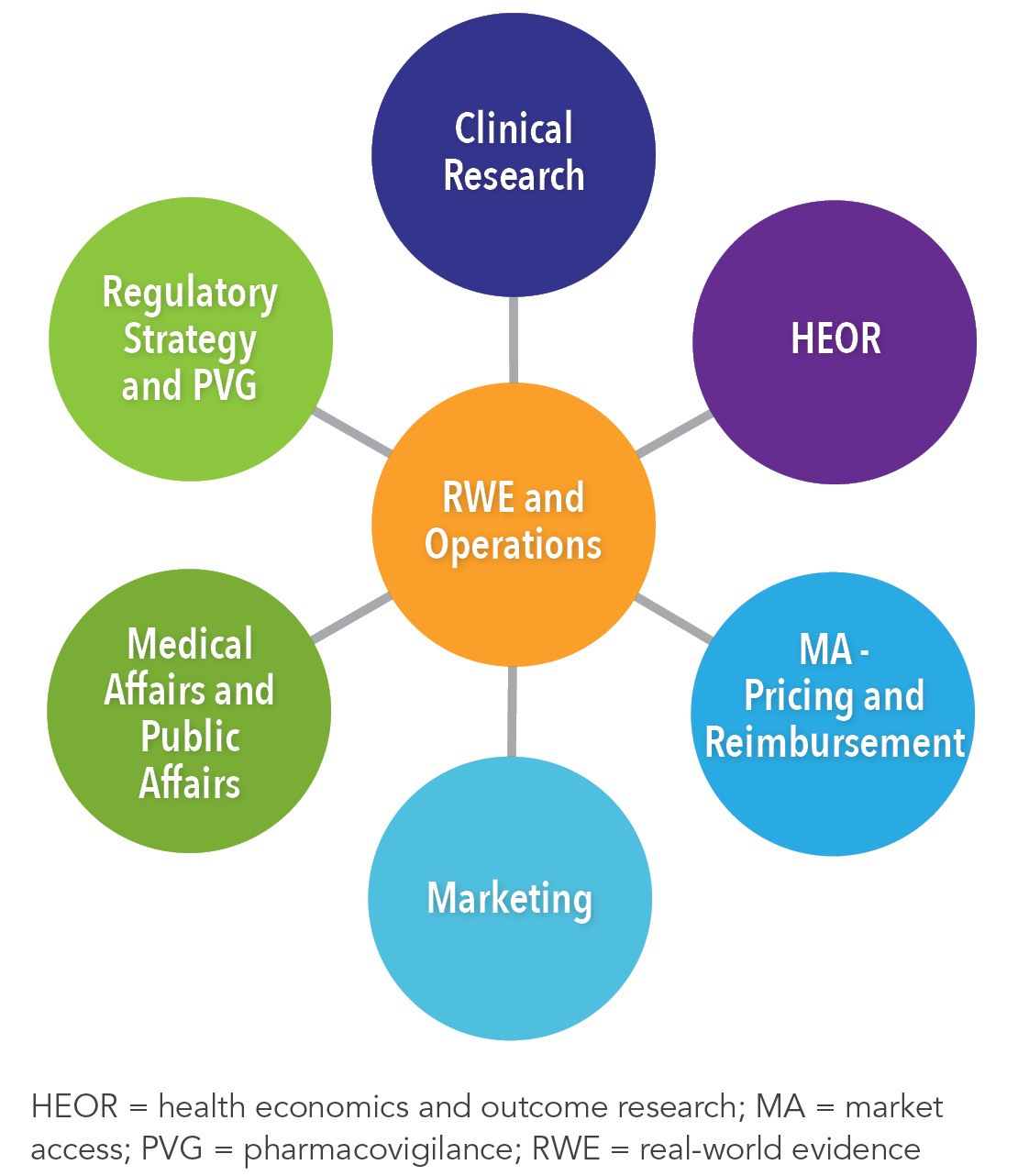
White Paper: Protocol Design in Real-World Evidence: The Indispensable Link Between Strategic Need and Study Execution - Evidera
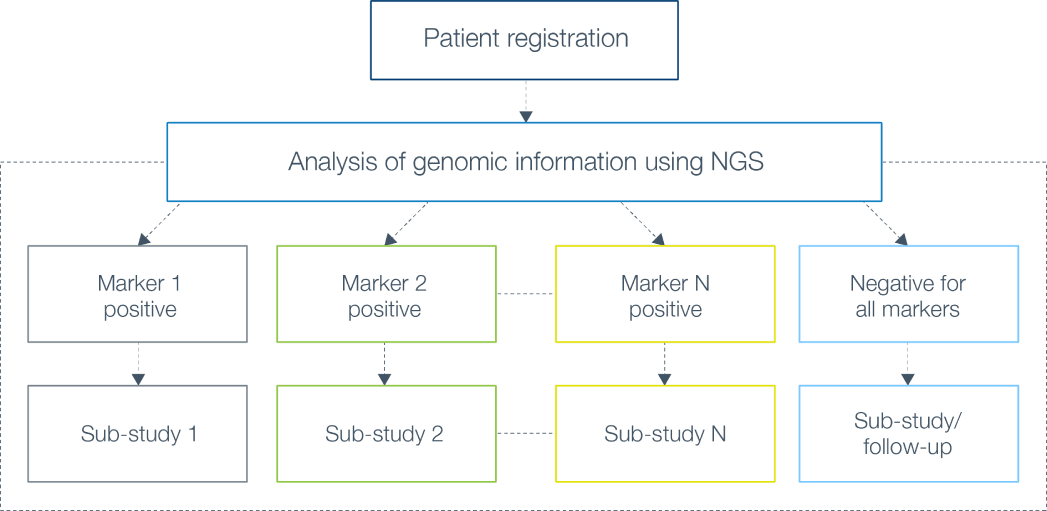
An interactive retrieval system for clinical trial studies with context-dependent protocol elements | PLOS ONE
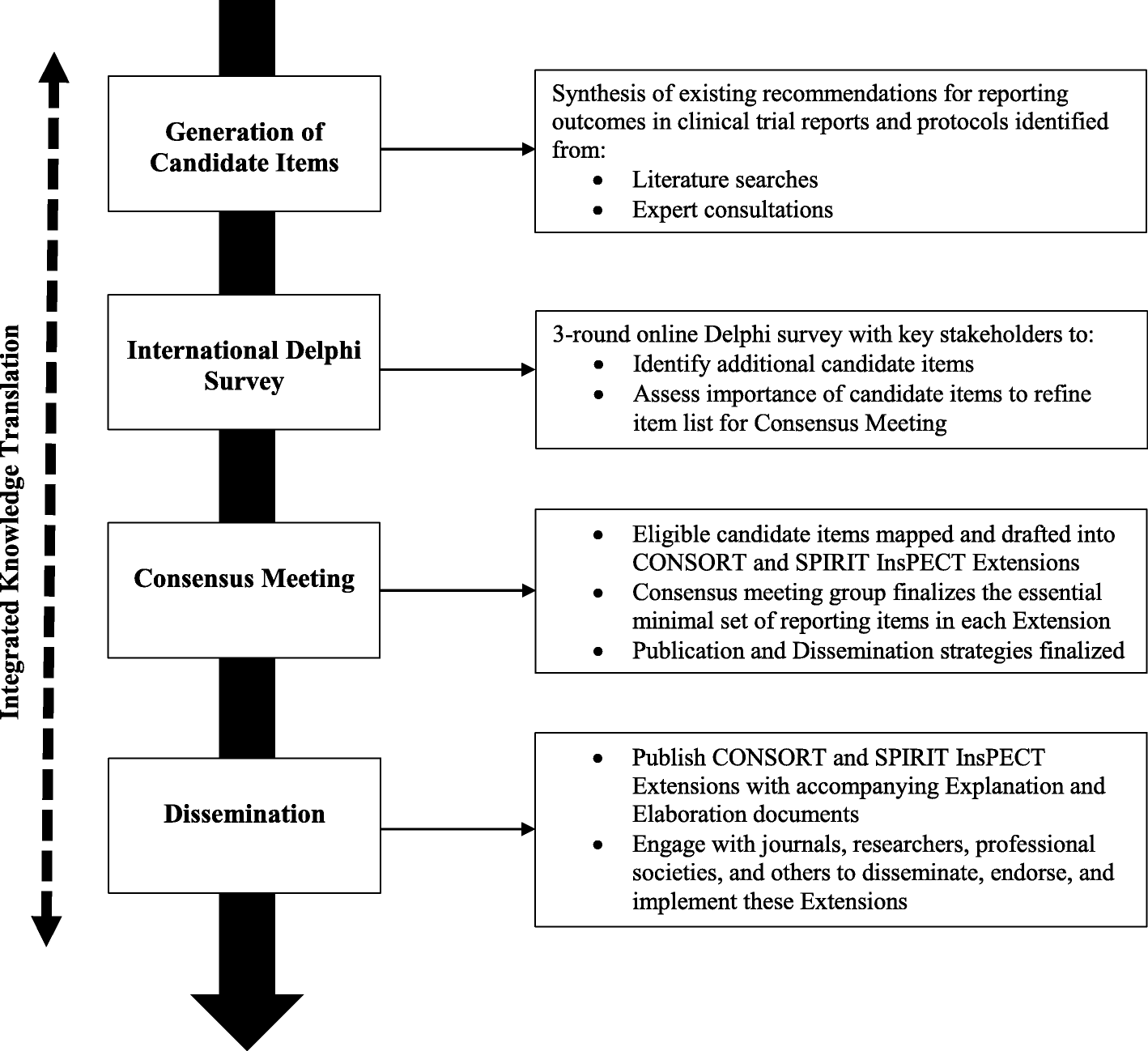
Improving outcome reporting in clinical trial reports and protocols: study protocol for the Instrument for reporting Planned Endpoints in Clinical Trials (InsPECT) | Trials | Full Text
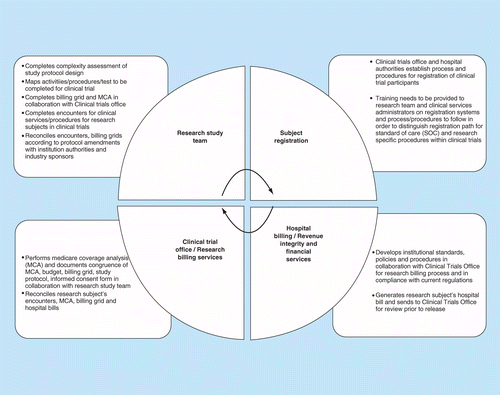
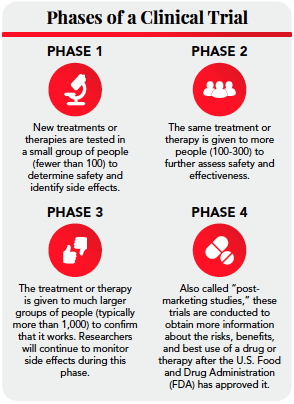
Optimization of protocol design: a path to efficient, lower cost clinical trial execution | Future Science OA