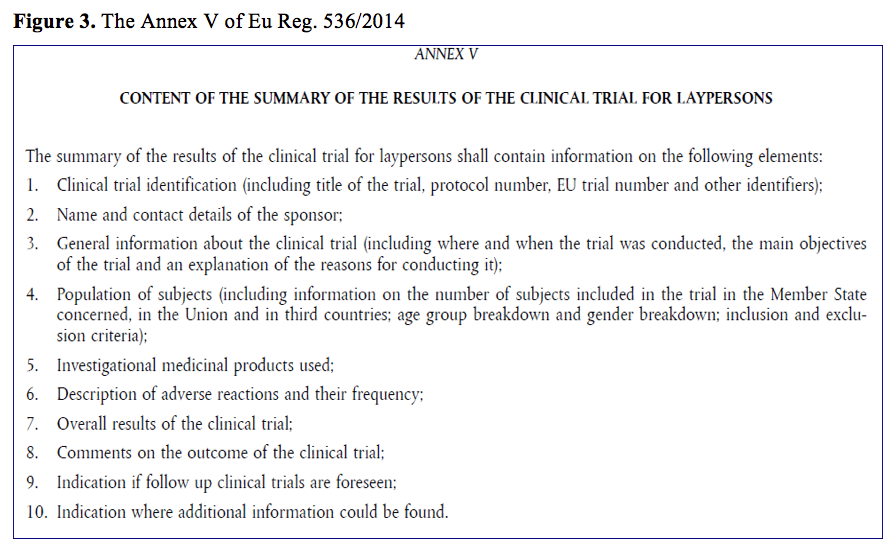
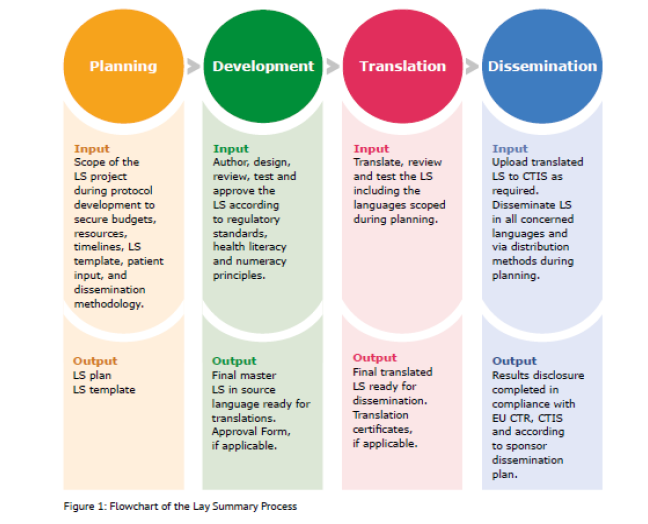
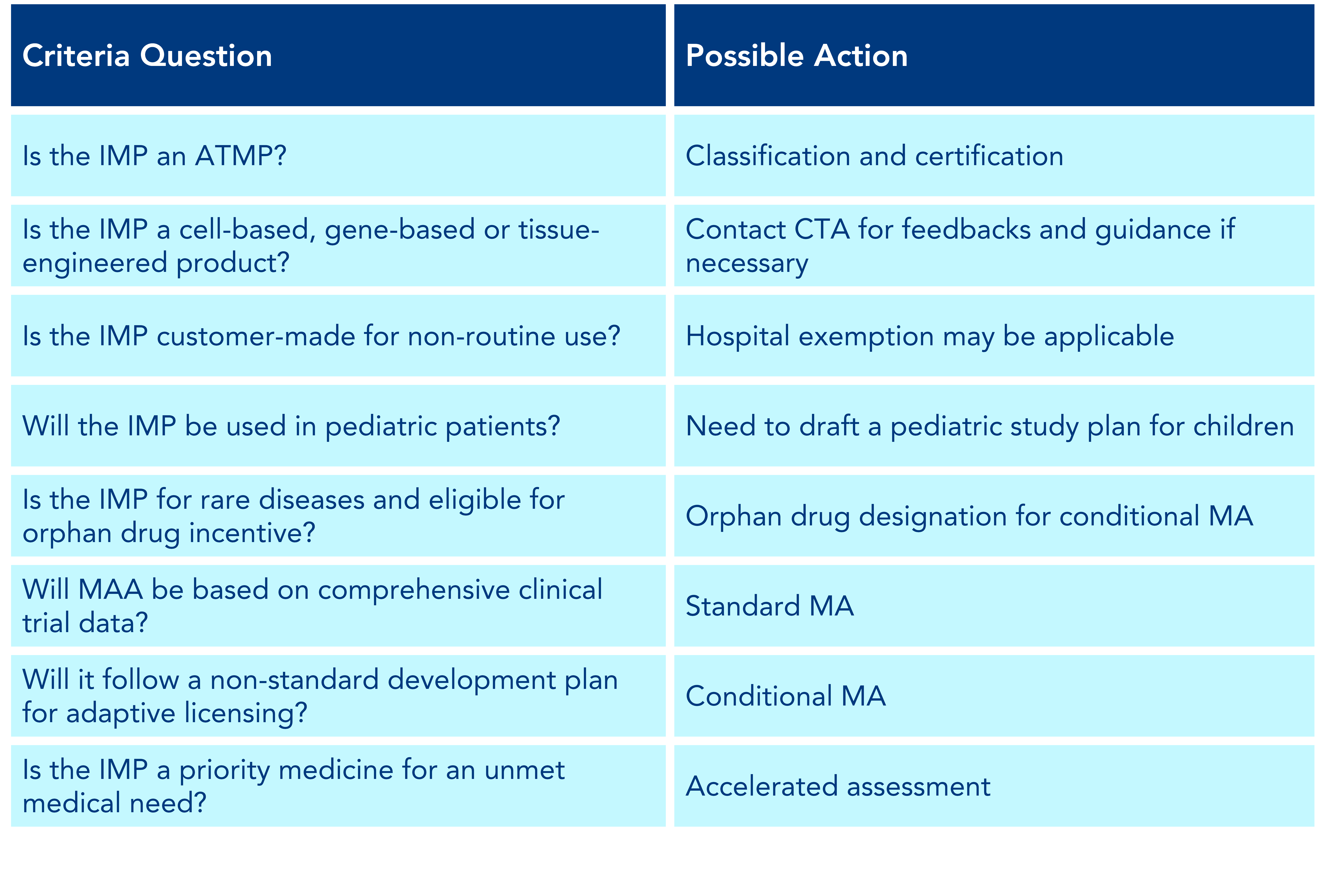
Pharmaceutics | Free Full-Text | Critical Analysis and Quality Assessment of Nanomedicines and Nanocarriers in Clinical Trials: Three Years of Activity at the Clinical Trials Office
EudraLex The Rules Governing Medicinal Products in the European Union Volume 4 Good Manufacturing Practice Guidelines on Good M