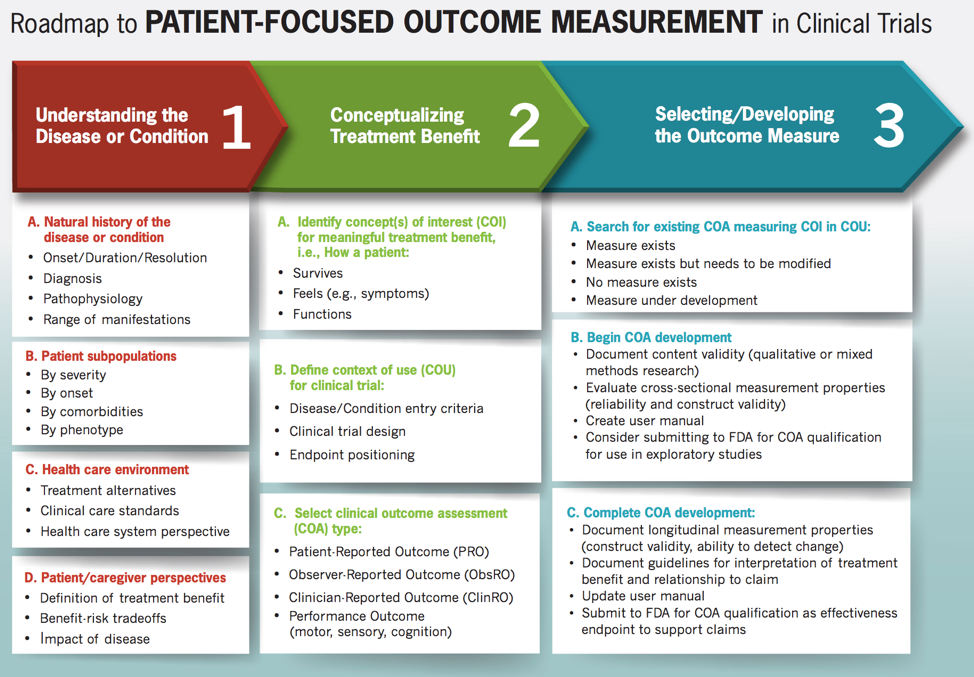
Patient-Focused Drug Development: Selecting, Developing, or Modifying Fit-for-Purpose Clinical Outcome Assessments Snapshot

Book 13: 2023 FDA Guidance on Clinical Study Reports and Statistical P – Clinical Research Resources, LLC
FDA Guidance on Conduct of Clinical Trials of Medical Products during COVID-19 Public Health Emergency Guidance for Industry, In