Form FDA 3455 - Disclosure: Financial Interest and Arrangements of Clinical Investigators Free Download

INVESTIGATOR RESPONSIBILITIES April Objectives Review and Discuss: Responsibilities of the clinical research Investigator as per relevant regulations. - ppt download
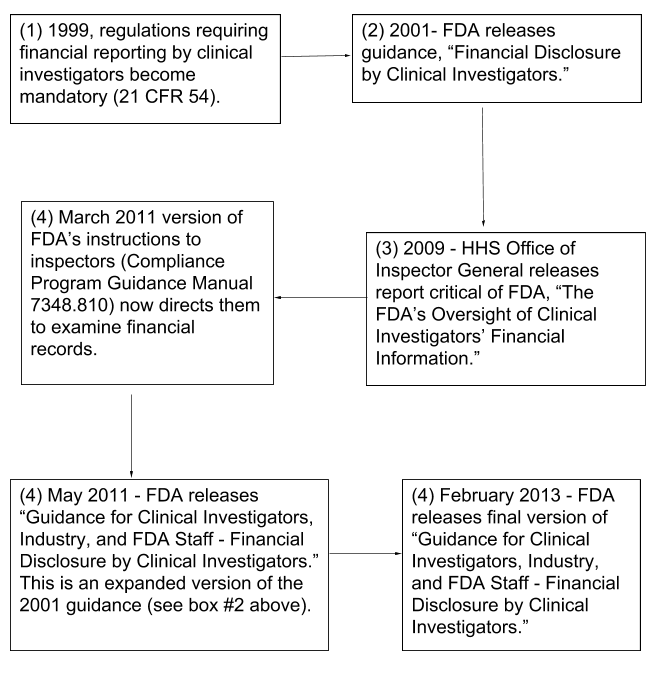
Process for collection of financial disclosure by clinical investigators per 21 CFR 54.4 Background: U.S. regulations, 21 CFR 32