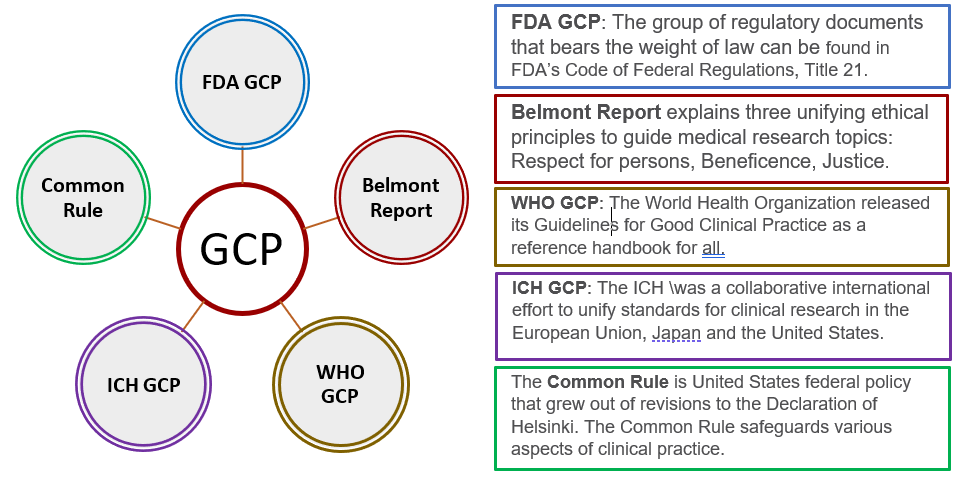
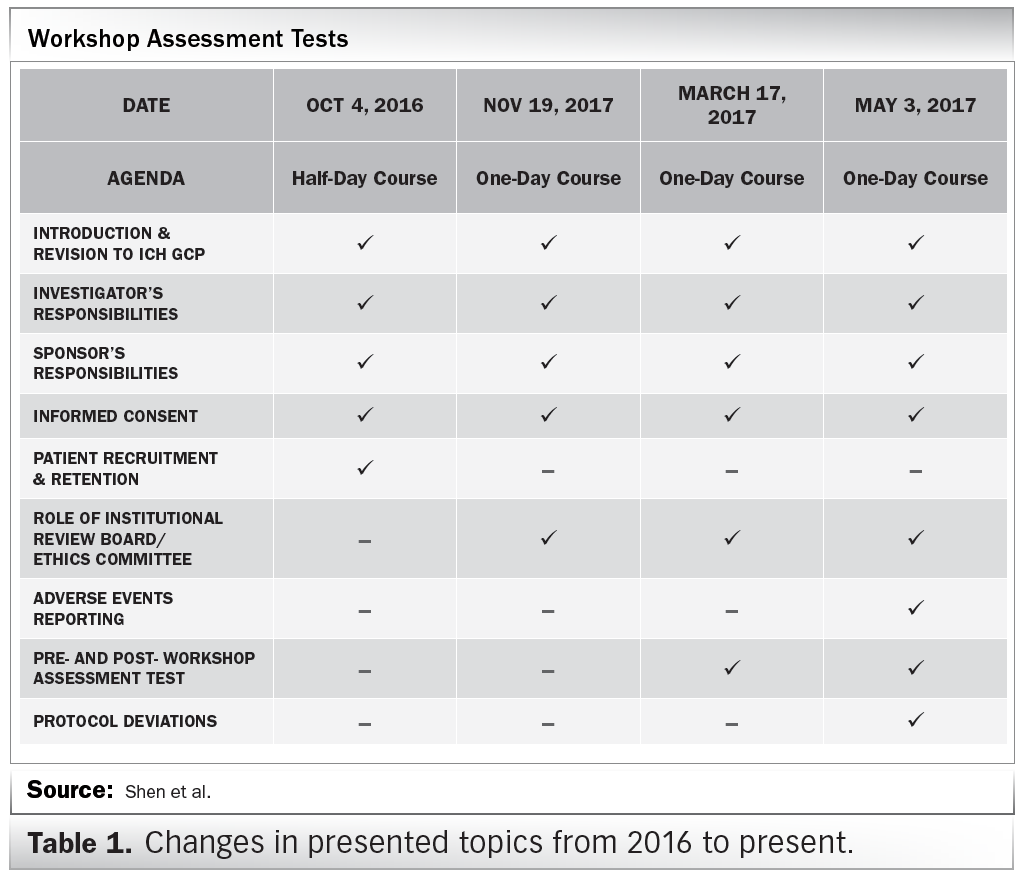
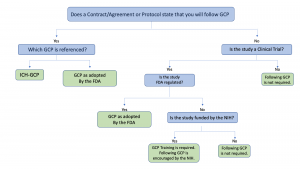
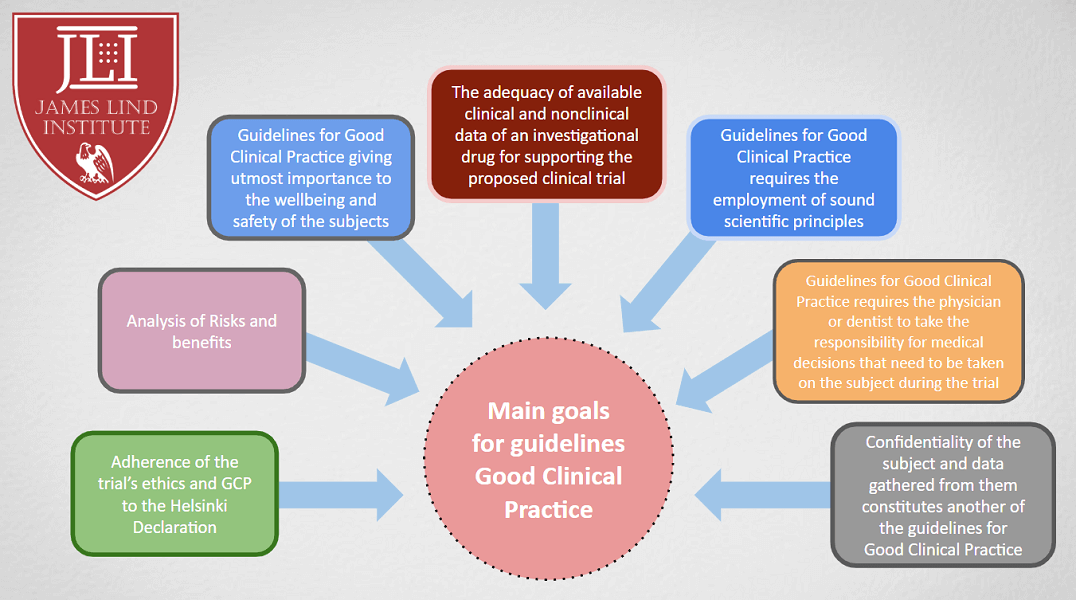
Principles of Good Clinical Practice (GCP) – What is it all about and who is responsible for adherence? GCP and QA All SIAC Call Mar 14, 2008 Munish Mehra, - ppt download

Compliance with the ICH-GCP Guidelines among the Saudi Health Care Professionals: Should Saudi Arabia Conduct Widespread ICH-GCP Training? | Semantic Scholar

The Good Clinical Practice guideline and its interpretation – perceptions of clinical trial teams in sub‐Saharan Africa - Vischer - 2016 - Tropical Medicine & International Health - Wiley Online Library

Whitehall Training - Want a handy guide explaining GCP guidelines and recent changes? Here's a snapshot of the GCP principles including recent amendments! Click the link to buy now: goo.gl/bK42I2 | Facebook

Clinical Trials: Study Design, Endpoints and Biomarkers, Drug Safety, and FDA and ICH Guidelines by Tom Brody PhD Dr. (2016-03-14): Amazon.com: Books