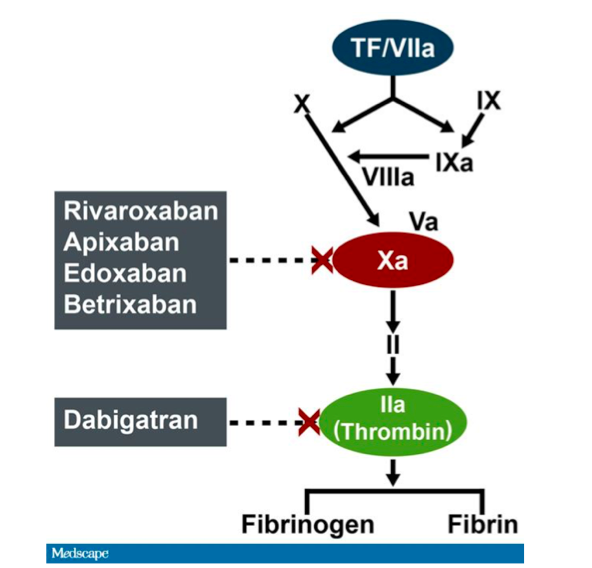
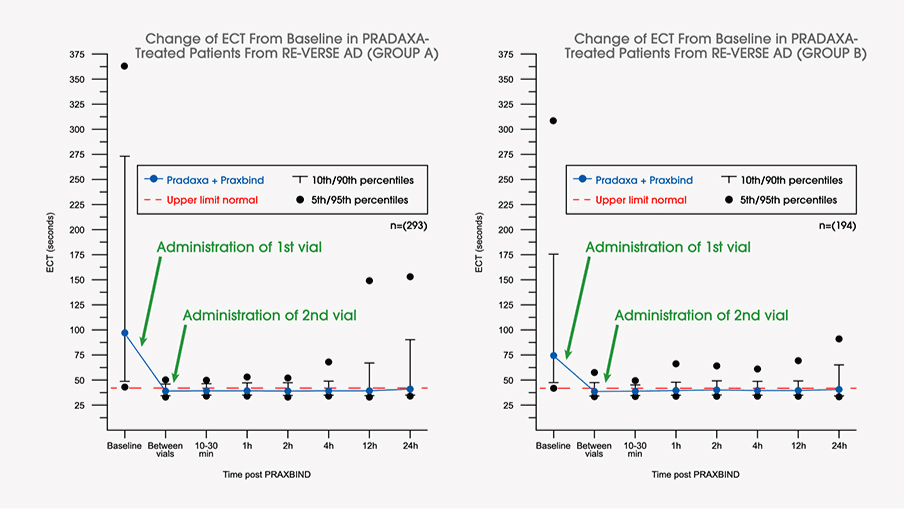
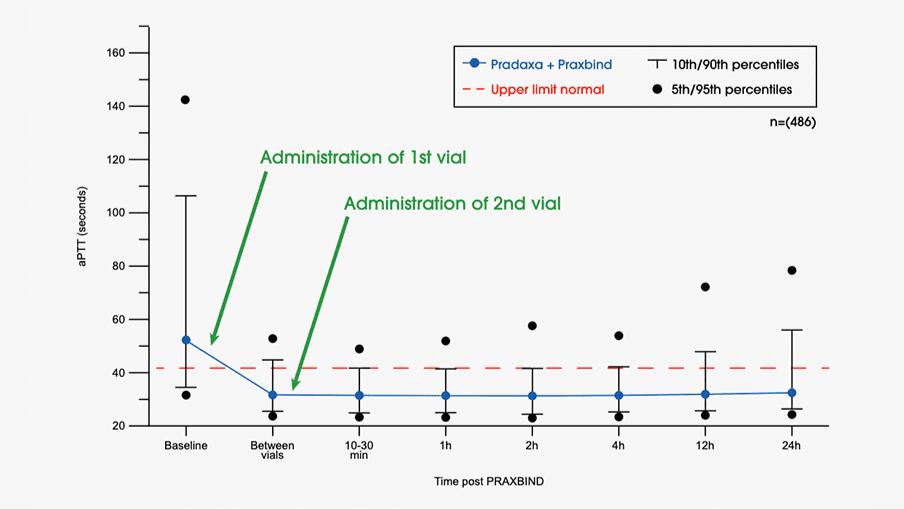
Dabigatran Reversal With Idarucizumab in Patients With Renal Impairment | Journal of the American College of Cardiology

Safety, tolerability, and efficacy of idarucizumab for the reversal of the anticoagulant effect of dabigatran in healthy male volunteers: a randomised, placebo-controlled, double-blind phase 1 trial - The Lancet

Idarucizumab for dabigatran reversal: the first 6 months in a tertiary centre - Wheeler - 2019 - Internal Medicine Journal - Wiley Online Library

Idarucizumab dosing in patients with excessive dabigatran body burden - British Journal of Anaesthesia

Idarucizumab, a Specific Reversal Agent for Dabigatran: Mode of Action, Pharmacokinetics and Pharmacodynamics, and Safety and Efficacy in Phase 1 Subjects - ScienceDirect

Idarucizumab and factor Xa reversal agents: role in hospital guidelines and protocols - ScienceDirect
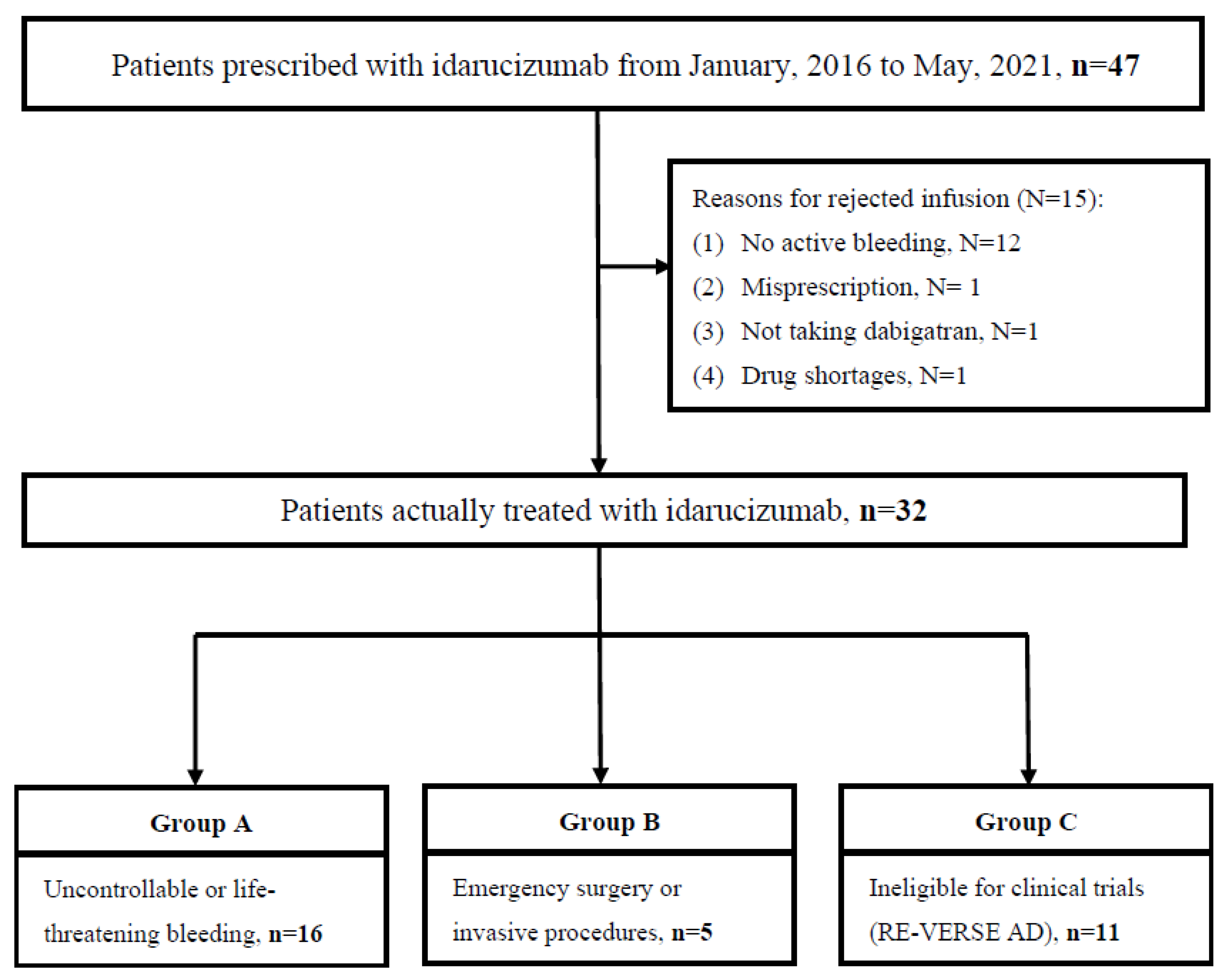
Medicina | Free Full-Text | Effectiveness and Safety of Dabigatran Reversal with Idarucizumab in the Taiwanese Population: A Comparison Based on Eligibility for Inclusion in Clinical Trials

Medicina | Free Full-Text | Effectiveness and Safety of Dabigatran Reversal with Idarucizumab in the Taiwanese Population: A Comparison Based on Eligibility for Inclusion in Clinical Trials
Usefulness of initial plasma dabigatran concentration to predict rebound after reversal | Haematologica
Interpretation of idarucizumab clinical trial data based on spontaneous reports of dabigatran adverse effects in the French phar

Safety, pharmacokinetics and pharmacodynamics of idarucizumab, a specific dabigatran reversal agent in healthy Japanese volunteers: a randomized study - Research and Practice in Thrombosis and Haemostasis

FDA Approves Praxbind® (idarucizumab), Specific Reversal Agent for Pradaxa® (dabigatran etexilate) | Business Wire