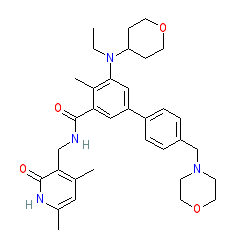
Epizyme Announces U.S. FDA Accelerated Approval of TAZVERIK™ (tazemetostat) for the Treatment of Patients with Epithelioid Sarcoma | Business Wire

Discovery of Dual Lysine Methyltransferase G9a and EZH2 Inhibitors with In Vivo Efficacy against Malignant Rhabdoid Tumor | Journal of Medicinal Chemistry

Cancers | Free Full-Text | Evaluation of Tazemetostat as a Therapeutically Relevant Substance in Biliary Tract Cancer
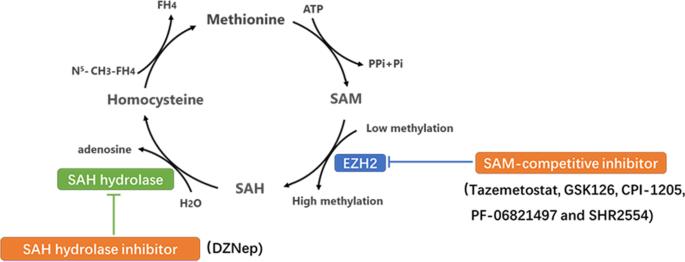
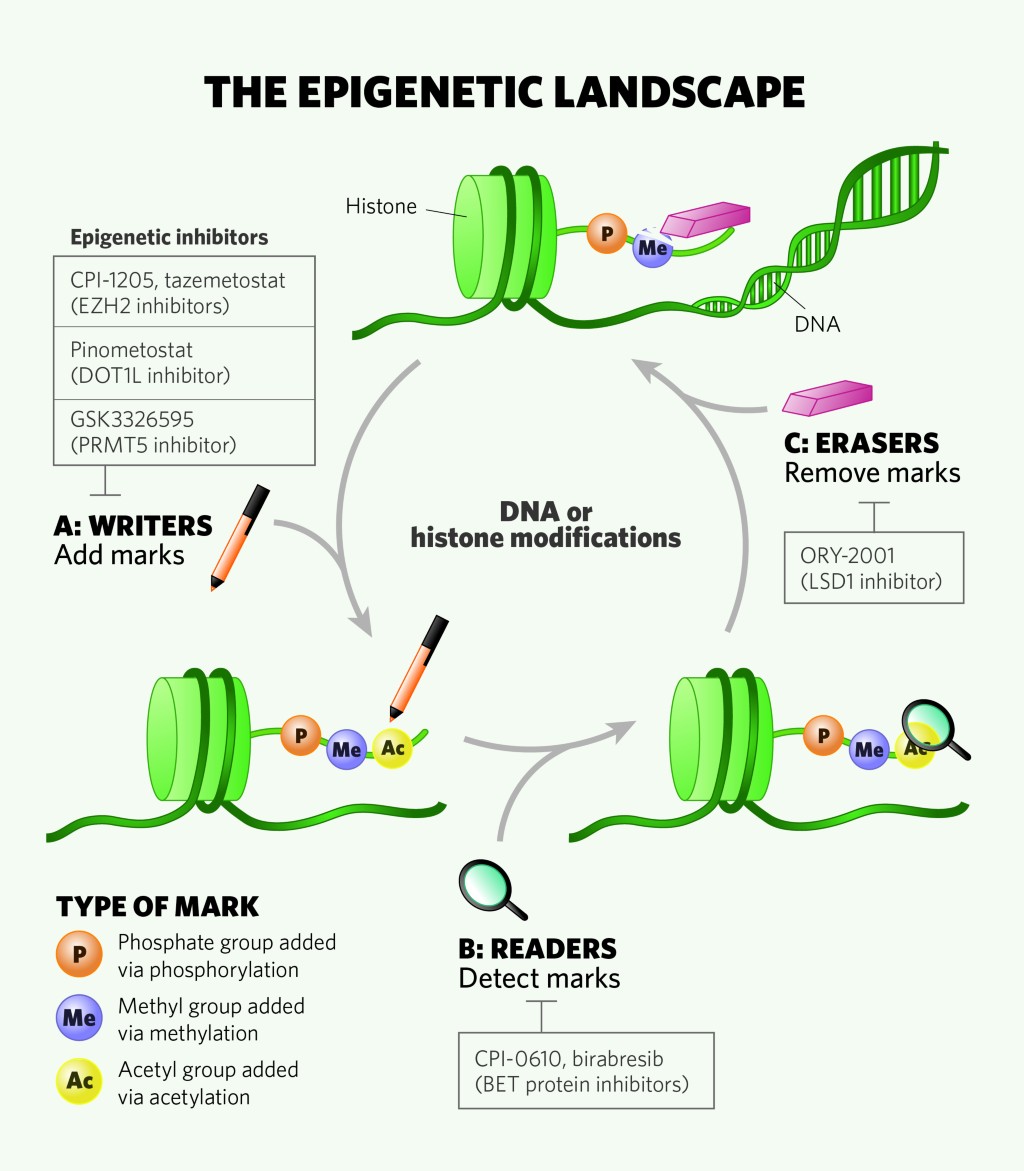
Finding an easy way to harmonize: a review of advances in clinical research and combination strategies of EZH2 inhibitors | Clinical Epigenetics | Full Text

Tazemetostat for patients with relapsed or refractory follicular lymphoma: an open-label, single-arm, multicentre, phase 2 trial - The Lancet Oncology
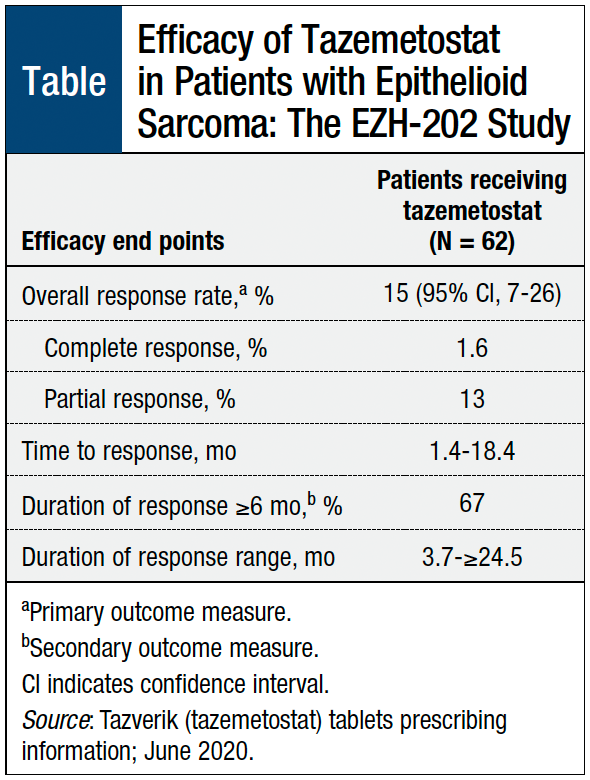
Tazverik (Tazemetostat) First FDA-Approved Treatment Specifically for Patients with Epithelioid Sarcoma
ANTICANCER AGENT “TAZVERIK® TABLETS 200mg” (TAZEMETOSTAT HYDROBROMIDE) LAUNCHED IN JAPAN FOR EZH2 GENE MUTATION-POSITIVE FOLLICULAR LYMPHOMA | News Release:2021 | Eisai Co., Ltd.

Epizyme Announces the U.S. Food and Drug Administration Lifts Partial Clinical Hold on Tazemetostat Clinical Program | Business Wire

The EZH2 inhibitor tazemetostat upregulates the expression of CCL17/TARC in B‐cell lymphoma and enhances T‐cell recruitment - Yuan - 2021 - Cancer Science - Wiley Online Library

Tazemetostat, an EZH2 inhibitor, in relapsed or refractory B-cell non-Hodgkin lymphoma and advanced solid tumours: a first-in-human, open-label, phase 1 study - The Lancet Oncology

Targeting Enhancer of Zeste Homolog 2 for the Treatment of Hematological Malignancies and Solid Tumors: Candidate Structure–Activity Relationships Insights and Evolution Prospects | Journal of Medicinal Chemistry

Tazemetostat for patients with relapsed or refractory follicular lymphoma: an open-label, single-arm, multicentre, phase 2 trial - The Lancet Oncology

Testing the Addition of Tazemetostat to the Immunotherapy Drug, Pembrolizumab (MK-3475), in Advanced Urothelial Carcinoma